GEH Network: Discerning Data: Gender, Power, and the Politics of Evidence
9 July 2025 | GENDRO
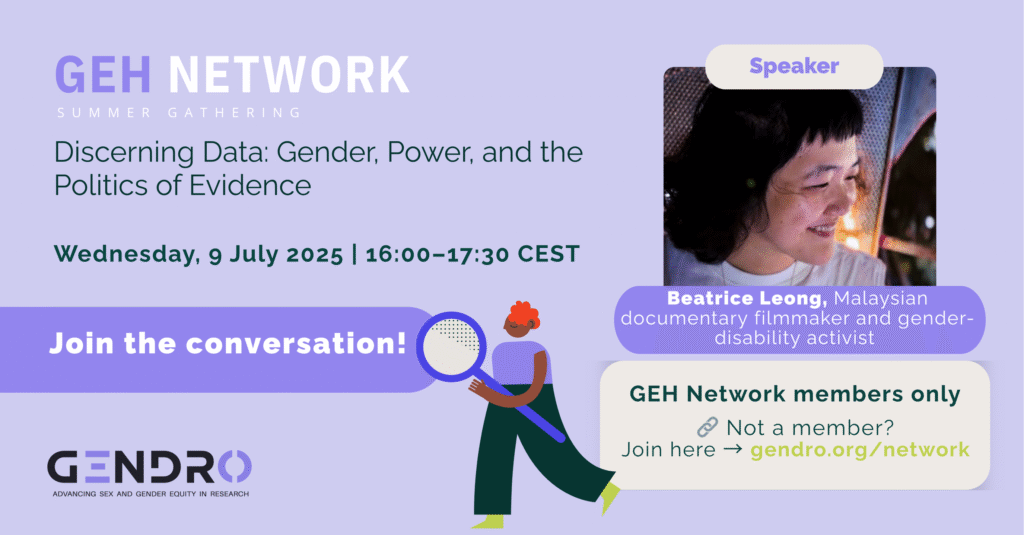
Who gets to decide what counts as truth? Why are certain bodies—especially gender nonconforming and disabled ones—so often reduced to data points, risks, or problems to fix? I’ll share some insights and tracing some links between gender conversion therapy and behaviourist models agenda still used today, and reflect on how data is being used to shape policy in ways that silence resistance. Autism shows up as a trending flashpoint recently, but the bigger story is about power, control, and how we might start reclaiming evidence on our own terms.
Speaker : Beatrice Leong is a Malaysian documentary filmmaker and gender-disability activist who believes in the power of stories to name what others silence. When she was finally diagnosed with autism in adulthood, after a lifetime of psychiatric and medical injustice, she began using storytelling to reclaim what was lost and connect with others who’ve been left out. Alongside filmmaking, she works across national, regional, and global spaces to advance disability inclusion, policy change, and narrative justice. Beatrice also serves on the advisory board of the Disability Justice Project, a media platform that supports disabled human rights defenders in the Global South through storytelling and advocacy.
Not a member? Join here
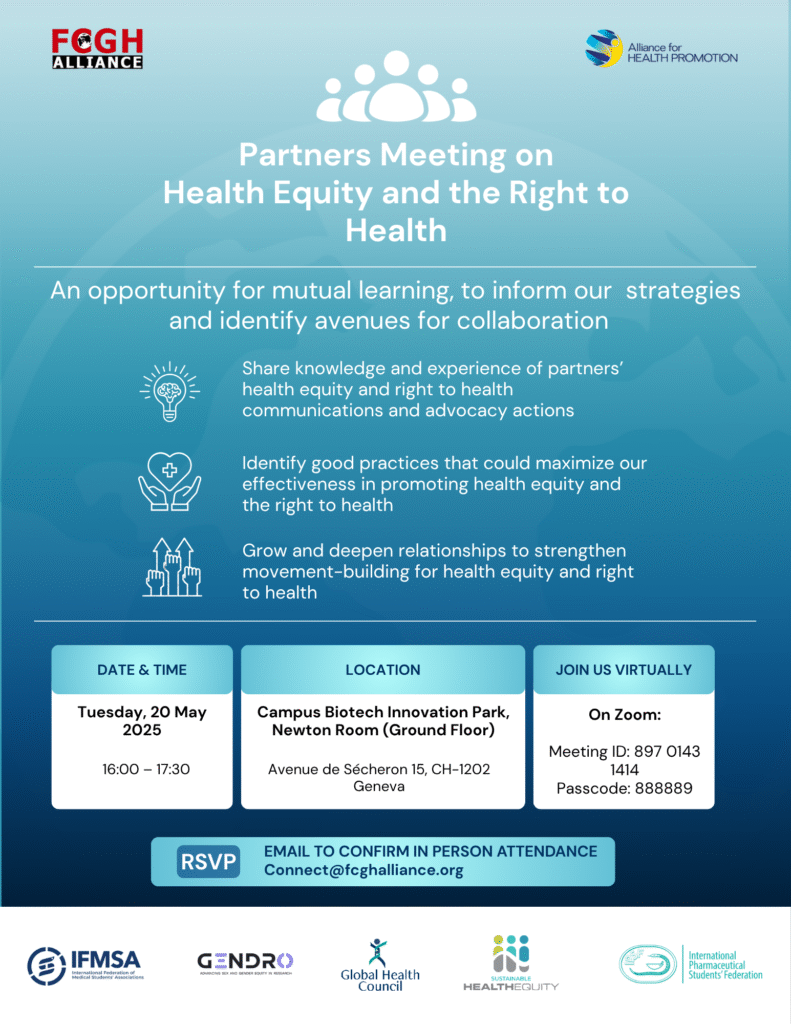
Partners Meeting on Health Equity and the Right to Health
20 May 2025 | Organizer: Framework Convention on Global Health Alliance and the Alliance for Health Promotion
GENDRO is proud to co-sponsor the WHA78 Partners Meeting on Health Equity and the Right to Health, held at Campus Biotech Innovation Park in Geneva. Co-organized by the Framework Convention on Global Health (FCGH) Alliance and the Alliance for Health Promotion, the event brought together civil society organizations and global health advocates to strengthen collaboration and advance the right to health for all.
Moderated by Dr. Leigh Kamore Haynes (Simmons University / FCGH Alliance), the meeting featured a dynamic exchange on the structural determinants of health inequities and strategies for collective action.
We thank our co-sponsors and all participants for their commitment to building a more equitable future.
GEH Network: Reimagining Research: Intersectional Feminist Approaches for Just Evidence
6 May 2025 | GENDRO

At Gendro, we convened an informal, closed online panel with members of the GEH Network to explore a timely and essential question: Do we need a new paradigm to conduct research?
With a focus on gender responsive /intersectional feminist research, the aim of this session was to create an interactive and safe space for open reflection on how recent social, political, and funding developments are affecting research on the intersection of health, gender, race/ethnicity, and other intersectional dimensions. We brought together a diverse group of voices and experiences, including those working on intersectional feminist and inclusive methodologies, fostering collaborative and participatory approaches.
We were honored to have Olabukunola Williams, Sexual and Reproductive Health and Rights Lead at Akin Mama wa Afrika, moderating this panel, alongside other insightful contributors Farah Abdi (TGEU — Trans Europe and Central Asia ) and Derek M. Griffith, Ph.D. (University of Pennsylvania).

A proposed framework for monitoring and evaluating progress at the intersection of women, power, and cancer
Elise M Garton, Gavin Allman, Hyo Sook Bae, Kalina Duncan, Ibithal Fadhil, Nazik Hammad, Shirin Heidari, Meritxell Mallafré-Larrosa, Jennifer Moodley, Rachel Nugent, Isabelle Soerjomataram, Carolyn D Taylor, Karla Unger-Saldaña, Verna Vanderpuye, Ophira Ginsburg.
The Lancet – April 15, 2025
The Lancet Commission on women, power, and cancer,1 hereafter referred to as the Commission, was created to address urgent questions at the intersection of social inequality, cancer risk and outcomes, and the status of women in society. Cancer is an increasingly important public health threat and economic challenge to all people worldwide, but has a disproportionate impact on the lives and livelihoods of women, which creates downstream impacts for society. The Commission applied an intersectional feminist lens2 to inform a nuanced, evidence-based, gendered approach to cancer risk and cancer control in response to this threat. The Commission report was published in September, 2023, with ten key findings and corresponding priority recommendations directed at a broad range of stakeholder communities: international organisations, national and subnational governments, researchers and research funders, civil society, and the private sector.1 To increase the likelihood that the recommendations set out in the Commission will be adopted and operationalised by multiple stakeholders, and to support the uptake of these recommendations, the authors proposed a framework and set of key performance indicators to guide implementation and to increase engagement of the global community at the nexus of gender, power, and cancer.
